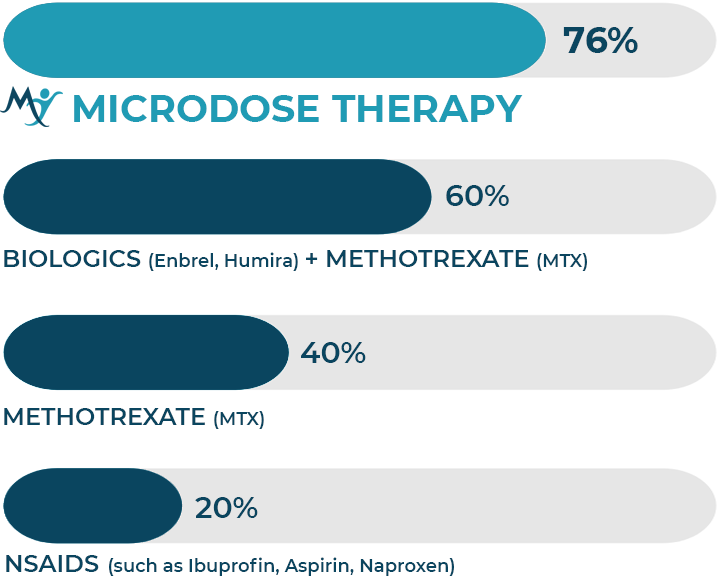
MICRODOSE THERAPY WORKS
Microdose Therapy™ has reduced symptoms by 76% in over 2600 patients. The results of a 2400 person study were published in the Journal of Inflammation Research in 2019. The study participants had 37 different inflammatory diseases including Arthritis, Osteoarthritis, Psoriatic Arthritis, Rheumatoid Arthritis, Bursitis, Chronic Fatigue Syndrome, Crohn’s Disease, Dementia, Eczema, Fibromyalgia, IBS, Parkinsons, Neuropathy and Vertigo. Although inflammatory symptoms vary by individual and disease, the symptom reduction included pain, stiffness, numbness, fatigue, brain fog, tingling, tremors, depression, and movement improved.












